Abstract
Introduction: Myeloproliferative neoplasms (MPN) are associated with an increased risk of clonal evolution to more aggressive myeloid disorders and the development of second malignancies (SM) including lymphoproliferative and solid tumors. The reasons for SM are unclear, but a therapy-related effect has been invoked. Ruxolitinib (RUX) is a guideline-recommended cytoreductive agent used in the US for myelofibrosis (MF) and polycythemia vera (PV) since 2011 and 2014, respectively. RUX has been linked to an increased risk of non-melanoma skin cancers (NMSC), but its influence on the development of other SM is debated. We aimed to evaluate the effect of RUX on the development of solid and lymphoid SM in a large, population-based cohort of patients (pts) with MPN.
Methods: We conducted a retrospective cohort study of older adults with MPN including PV, essential thrombocythemia (ET) and MF in the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. Pts were required to 1) be diagnosed in 2012-2017, 2) be 66-99 years of age at diagnosis, 3) have continuous enrollment in Medicare Parts A and B from one year before MPN diagnosis through end of follow-up, and 4) have continuous Part D coverage from diagnosis to the end of follow-up. We excluded pts who were health maintenance organizations members, or treated with chlorambucil, busulfan or allogeneic stem cell transplant. We followed pts from MPN diagnosis to 12/31/2019, SM diagnosis, death or change of insurance status, whichever came first. RUX proportion of days covered (PDC) was calculated as the ratio of the number of days the patient was covered by RUX to the number of days from RUX initiation to 6 months before end of follow-up. Our outcome of interest was first SM, i.e., any new malignancy other than new myeloid malignancy and NMSC, diagnosed at least 6 months after cohort entry. We identified SM using SEER data for the years 2012-2017 and Medicare claims for the years 2018-2019. We categorized SM as solid or lymphoid. The cumulative incidence function (CIF) of SM was computed via a competing risk model. Death and development of a SM other than malignancy of interest (solid or lymphoid) were considered as competing events. Gray's test was utilized to assess difference across strata. The impact of RUX on the development of SM was assessed by multivariable competing risks models. The models adjusted for age at MPN diagnosis, sex, race, marital status, SEER region, Elixhauser comorbidity score, history of prior malignancy, disability status, state buy-in, census tract Yost index and hydroxyurea exposure. All statistical tests were two-sided and performed with SAS Version 9.4.
Results: The study included 4019 pts (1556 PV, 1896 ET, 567 MF) with a median age at diagnosis of 77 (interquartile range [IQR]: 71-83) years. The median follow-up was 3.5 (IQR: 2.23- 4.98) years and 3.18 (IQR: 2.17- 4.75) years for 408 RUX users and 3611 non-users, respectively. Among RUX users, RUX was initiated after a median of 0.66 years since diagnosis. Pts received RUX for a median of 1.15 (IQR: 0.42-2.12) years with a median PDC of 94.6% (IQR: 71.3- 99.7%). Compared with those who did not receive RUX, RUX users were more likely to be younger, have MF and a history of prior malignancy.
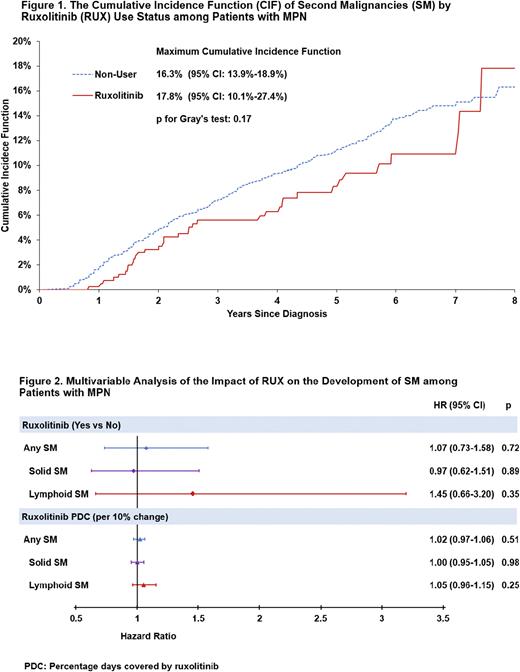
A total of 396 pts developed a SM including 306 solid SMs and 90 lymphoid SM. About 9% RUX users and 10% non-users developed a SM, resulting in a CIF of 17.8% (95% confidence interval [CI]: 10.1-27.4%) and 16.3% (95% CI: 13.9-18.9%), respectively (Gray's test, p=0.17; (Figure 1). The CIF of solid SM among RUX users was 11.8% (95% CI: 5.4-21.1%), and 13.0% among non-users (95% CI: 10.7-15.5%, p= 0.06); in lymphoid SM, CIF was 6.0 % (95% CI: 2.64-11.3%) among RUX users, and 3.3% (95% CI: 2.6- 4.2%) among non-users (p=0.52).
In the multivariable model, the risk of any SM (hazard ratio [HR]=1.07, 95% CI: 0.73-1.58, p=0.72), solid (HR=0.97, 95% CI: 0.62-1.51, p=0.89) and lymphoid SM (HR=1.45, 95% CI: 0.66-3.20, p=0.35) did not differ by RUX use status. No difference in the risk of any SM was observed when evaluating the influence of RUX PDC (Figure 2) Similar findings were seen in MPN subgroups.
Conclusions: In this large population-based study of older pts with MPN, the risk of solid and lymphoid SM was similar among RUX users and non-users. Our results suggest that the use of RUX does not increase the risk of SM in older pts with MPN.
Acknowledgments: The study was supported by Frederick A. Deluca Foundation.
Disclosures
Wang:Celgene: Research Funding. Shallis:Bristol Myers Squibb and Gilead Sciences, Inc: Honoraria; Gilead Sciences, Inc.: Honoraria. Huntington:Agios: Research Funding; Janssen: Consultancy; Genentech: Consultancy; AbbVie: Consultancy; FlatonIron Health: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy; ADC Therapeutics: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Merck: Consultancy; Seattle Genetics: Consultancy, Honoraria; Tyme: Consultancy; Pharmacyclics: Honoraria; AstraZeneca: Consultancy; Arvinas, Novartis, Servier, Bayer, SeaGen: Consultancy; Debiopharm Group: Research Funding; TG Therapeutics: Consultancy, Research Funding; DTRM Biopharm: Research Funding; Celgene: Research Funding. Zeidan:Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board; Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards. Neparidze:Janssen: Research Funding; GSK: Research Funding. Ma:Bristol Myers Squibb: Consultancy. Podoltsev:Incyte: Honoraria; Agios Pharmaceuticals: Honoraria; Pfizer: Honoraria; Cogent Biosciences: Other: Independent Data Review Committee ; AbbVie: Honoraria; Blueprint Medicines: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; CTI BioPharma: Honoraria; PharmaEssentia: Honoraria; Novartis: Honoraria; Constellation Pharmaceuticals: Honoraria.
OffLabel Disclosure:
Ruxolitinib, FDA-approved indication for treatment of polycythemia vera and myelofibrosis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal